CLINICAL TRIAL
This clinical trial is a Malaysia and China Government to Government (G2G) Initiative to conduct Phase 3 Clinical Trial COVID-19 Vaccine in collaboration with Ministry Of Health (MOH).
TOPPHARMA and IMBCAMS have mutually agreed to collaborate for the joint clinical project of Conducting the Phase 3 Clinical Trial Of Inactivated SARS-CoV-2 Vaccine in Malaysia. This clinical study protocol is a global multi-centre Phase 3 clinical trial and individually randomized, double-blinded, placebo-controlled study. The study is planned for assessing the safety and efficacy of the COVID-19 vaccine.
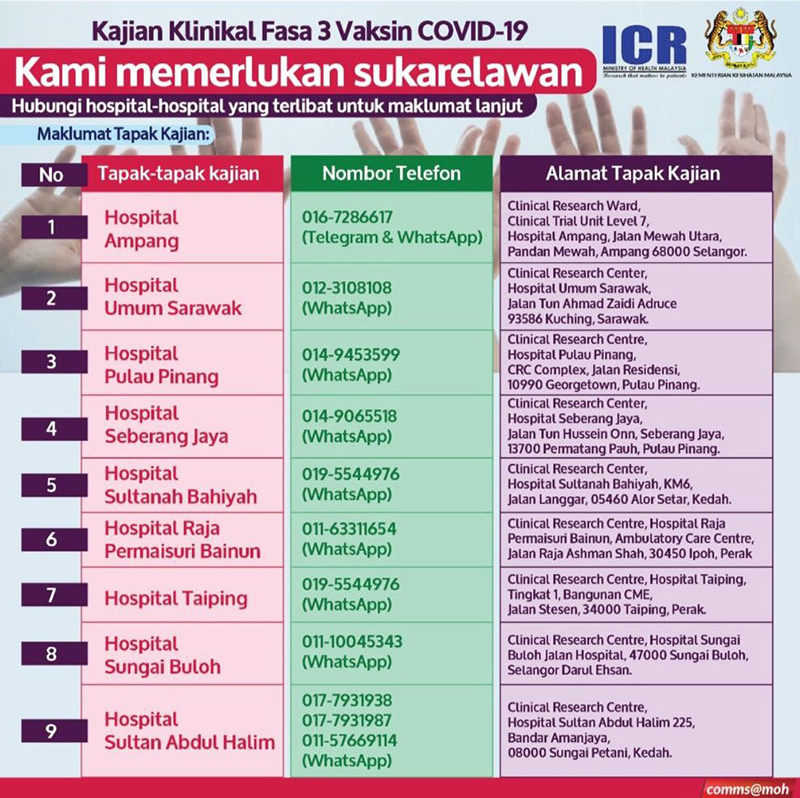
On 27th January 2021, MOH has official launched and started clinical trial recruitment of 3,000 volunteers from various races, religions and background to participate in this clinical trial study at nine Ministry of Health hospitals by their respective Clinical Research Centres (CRC). This is the first of its kind largest clinical trial conducted in Malaysia.
Clinical Trial Study in Malaysia
Malaysia and China continue to cooperate for the clinical trial of COVID-19 Phase 3 vaccine through the involvement of the Ministry of Health Malaysia (MOH) and sponsorship by the Institute of Medical Biology of the Chinese Academy of Medical Sciences (IMBCAMS). IMBCAMS has previously conducted Phase 1 and Phase 2 clinical trials of COVID-19 in China, while similar Phase 3 clinical trials is based on 'Global Multi-Centre' trial which is currently being conducted in Brazil, Mexico and Bangladesh.
Based on the results of Phase 1 and Phase 2 Clinical Trial, both studies have shown a significant safety profile of the COVID-19 vaccine where the side effect rate does not exceed 30% overall after 28 days of immunization with some common side effects such as pain, redness and itching at the injection site, fever, cough, fatigue, diarrhea, nausea, and allergic reactions.
The SARS-CoV-2 vaccine was evaluated in the clinical trial is based on an inactive vaccine platform made from a virus that had been killed through a physical or chemical process, and it had no potential to cause disease. The inactive vaccines usually do not confer as strong immunity as compared to live vaccines causing several doses to be required from time to time to build an immune response to COVID-19 disease. Examples of vaccines that use inactive viruses are Hepatitis A, influenza, polio and rabies vaccines.
The Phase 3 clinical trial of COVID-19 vaccine was approved by the MOH through the Medical Research and Ethics Committee (MREC) on 14th December 2020, and the National Pharmaceutical Regulatory Agency (NPRA) on 8th January 2021. The study vaccine had arrived in Malaysia site on 23rd January 2021 and subsequently was distributed to all clinical trial sites in stages starting from 27th January 2021. The first participants had recruited and joined the first cohort of study subject in February 2021.
A total of 9 hospitals across the country had participated under the coordination of the Institute of Clinical Research (ICR) with the involvement of 3,000 volunteers aged 18 and above. A total of 50% of the total volunteers had received two (2) doses of inactive vaccine while another 50% received 2 doses of placebo (a study product similar to the study vaccine but not containing the SARS-CoV-2 antigen). Recipients of the study vaccine or placebo were given two (2) doses over a 14 -day interval on the upper arm muscles and were required to attend a total of six (6) visits within two (2) months and to be followed up for 13 months.
Advertisements for this vaccine study were posted on MOH and ICR social media on 15th January 2021 along with frequently asked questions (FAQ) information. More than 7,000 members of the public had contacted the clinical trial site and expressed their interest in participating this clinical trial.


Press Statement
IMBCAMS PHASE 3 CLINICAL TRIAL EXTENDED CROSSOVER AND BOOSTER STUDY
IMBCAMS KAJIAN KLINIKAL FASA 3 EXTENDED CROSSOVER AND BOOSTER STUDY